WASHINGTON — The drug industry’s decade-spanning search for a female equivalent to Viagra took a major step forward Thursday, as government experts recommended approval for a pill to boost sexual desire in women.
The first-of-a-kind endorsement came with safety reservations, however, because of side effects including fatigue, low blood pressure and fainting.
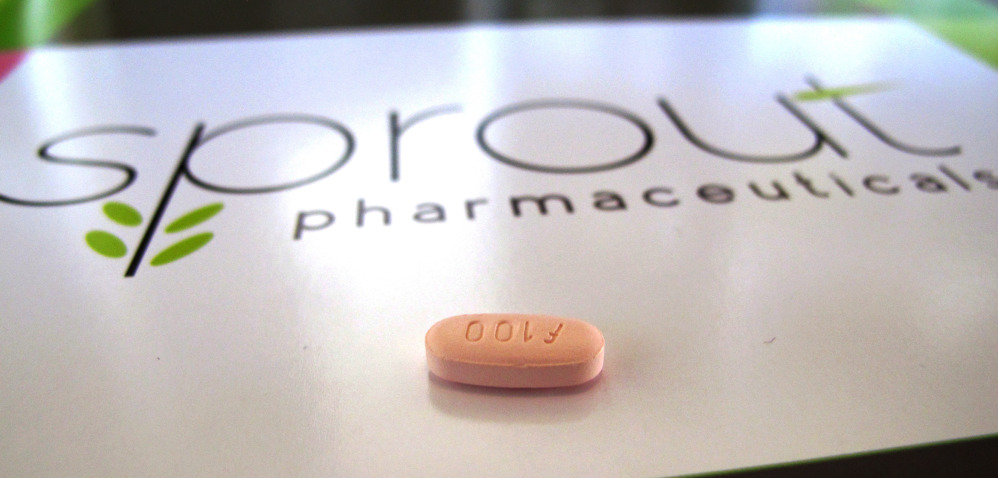
The panel of Food and Drug Administration advisers voted 18-6 in favor of Sprout Pharmaceutical’s daily pill, flibanserin, on the condition that the company develops a plan to manage its risks.
The recommendation is a major victory for a drug sometimes hailed as “female Viagra,” but which has been plagued for years by concerns of lackluster effectiveness and safety issues. The FDA has rejected the drug twice since 2010. And a similar panel of FDA experts voted unanimously against the drug five years ago.
Thursday’s vote is nonbinding but the FDA often follows the advice of its experts. An official decision is expected in August.
FDA’s experts acknowledged that flibanserin’s effect is not very strong, but said there is a need for FDA-approved drugs to address female sexual problems.
“These are very modest results,” said Dr. Julia Heiman of the Kinsey Institute at Indiana University. “But on the other hand, even modest results can make a lot of difference when you’re at a certain point in the clinical problem.”
In general, women taking flibanserin reported between 0.5 and 1 more sexually satisfying event per month, compared with women taking a placebo. They also scored higher on questionnaires measuring desire and scored lower on measures of stress.
Flibanserin, which acts on serotonin and other brain chemicals, was originally studied as an antidepressant, but then repurposed as a libido pill after women reported higher levels of sexual satisfaction.
The effort to trigger sexual interest through brain chemistry is the drug industry’s latest attempt to address women’s sexual problems.
Since the blockbuster launch of Pfizer’s Viagra in 1998, dozens of therapies have been studied for so-called female sexual dysfunction. But problems with women’s sexual desire have proven resistant to drugs that act on blood flow, hormones and other simple biological functions.
Flibanserin would be labeled for premenopausal women with hypoactive sexual desire disorder.
Send questions/comments to the editors.

Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.