SILVER SPRING, Md. — Government advisers dealt a blow Thursday to Philip Morris International’s hopes to sell its heat-not-burn device in the United States as a less-harmful alternative to cigarettes.
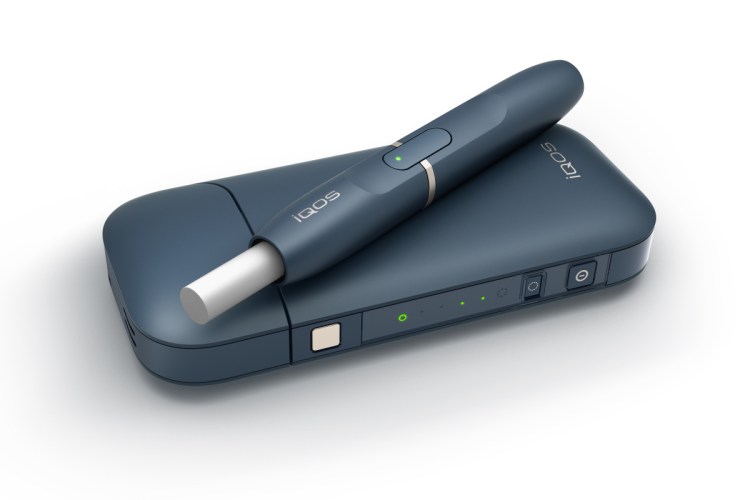
The penlike device heats Marlboro-branded sticks of tobacco but stops short of burning them. It is already sold in more than 30 countries and Philip Morris aims to make it the first “reduced risk” tobacco product ever sanctioned by the U.S.
The votes Thursday by the panel of Food and Drug Administration advisers on the marketing of the iQOS device are nonbinding. The FDA will make a separate decision on whether to allow the product on the market, and – if so – how it could be marketed to consumers.
FDA clearance would mark a major milestone in efforts by both the industry and government officials to provide alternative tobacco products to U.S. smokers. The adult smoking rate has fallen to an all-time low of 15 percent, though smoking remains the nation’s leading preventable cause of illness and death.
According to the company, the heat-not-burn approach reduces exposure to tar and other deadly byproducts of cigarettes.
But panelists expressed doubts about the company’s studies.
Send questions/comments to the editors.


Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.