WASHINGTON — The Food and Drug Administration on Wednesday approved a device aimed at helping obese people shed weight in a novel way – by targeting the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness.
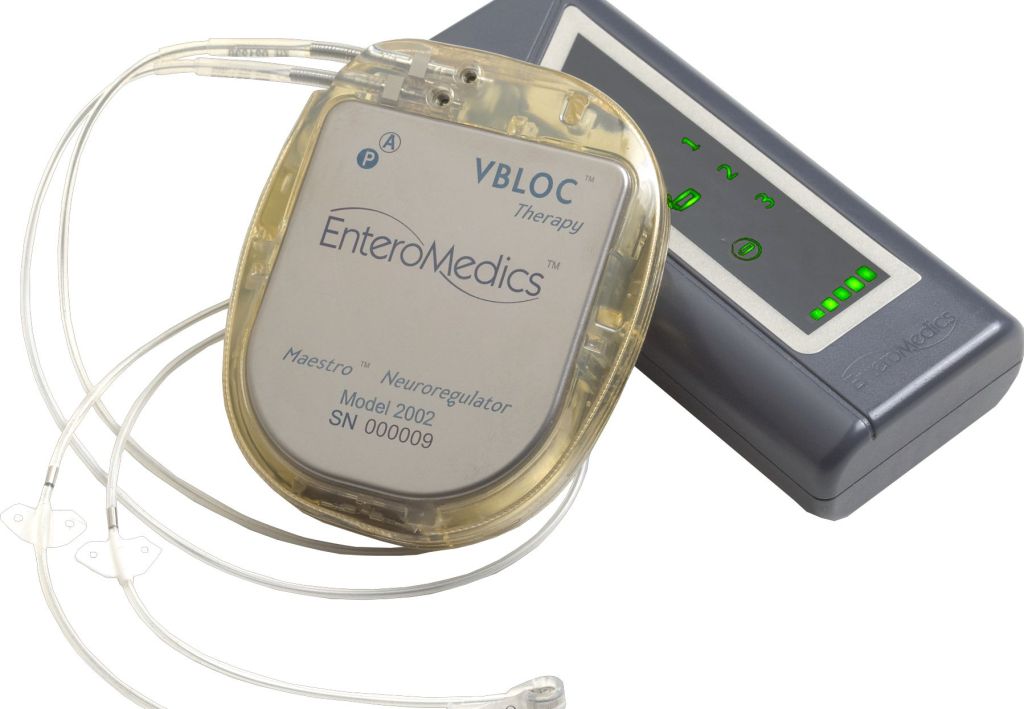
The Maestro Rechargeable System, as it is known, consists of an electrical charge generator, wire leads and electrodes that are implanted surgically into a patient’s abdomen. It sends electrical pulses designed to interfere with the vagus nerve, which signals to the brain when the stomach is full or empty.
Though researchers don’t know exactly how such electrical stimulation leads to weight loss, the approach seems promising. In a yearlong clinical trial involving 233 patients with a body-mass index, or BMI, of 35 or greater, those who received a working Maestro device lost 8.5 percent more weight than those without it. About half those in the experimental group lost at least 20 percent of their excess weight, and more than a third lost more than 25 percent of their excess weight.
The overall figure was below the original goal of the trial, which was to show weight loss of 10 percent more excess weight in the control group than in those using the new device. Nevertheless, an FDA advisory group said the data showed sustained weight loss among participants and argued that the benefits of the device outweigh its risks for certain patients. In the clinical trial, some patients experienced nausea, vomiting, surgical complications and other side effects. The FDA is requiring the device’s manufacturer, EnteroMedics, to conduct a five-year, post-approval study to gather additional data about its safety and effectiveness.
The device represents the first obesity device the FDA has approved since 2007, and the first to target the nerve signals that tell us when we are hungry or full. But it isn’t intended for people hoping to shed a few pounds in the new year.
To the contrary, the agency approved the pacemaker-like device for people over 18 who have not been able to lose weight through a weight-loss program, who have a BMI between 35 and 45 (normal is considered below 25) and who have at least one other obesity-related condition, such as type 2 diabetes.
In a statement Wednesday, EnteroMedics chief executive Mark Knudson called the approval a “transformational event” for obese people in search of a new and viable way to lose weight. “The Maestro System fills a significant gap in the currently available treatment options, offering clinically meaningful weight loss without the fear or many of the side effects associated with existing bariatric options,” he said.
More than a third of U.S. adults are obese, according to the Centers for Disease Control and Prevention. Obesity causes increased risk for heart disease, stroke, type 2 diabetes and certain types of cancer and, the CDC says, leads to an estimated $147 billion in medical costs a year.
Send questions/comments to the editors.


Success. Please wait for the page to reload. If the page does not reload within 5 seconds, please refresh the page.
Enter your email and password to access comments.
Hi, to comment on stories you must . This profile is in addition to your subscription and website login.
Already have a commenting profile? .
Invalid username/password.
Please check your email to confirm and complete your registration.
Only subscribers are eligible to post comments. Please subscribe or login first for digital access. Here’s why.
Use the form below to reset your password. When you've submitted your account email, we will send an email with a reset code.